We build products for Healthcare and Life Science companies to accelerate innovation and get to market faster.







The Sierra Labs Compliance Stack
Automation decreases time and effort for compliance and increases productivity and innovation.
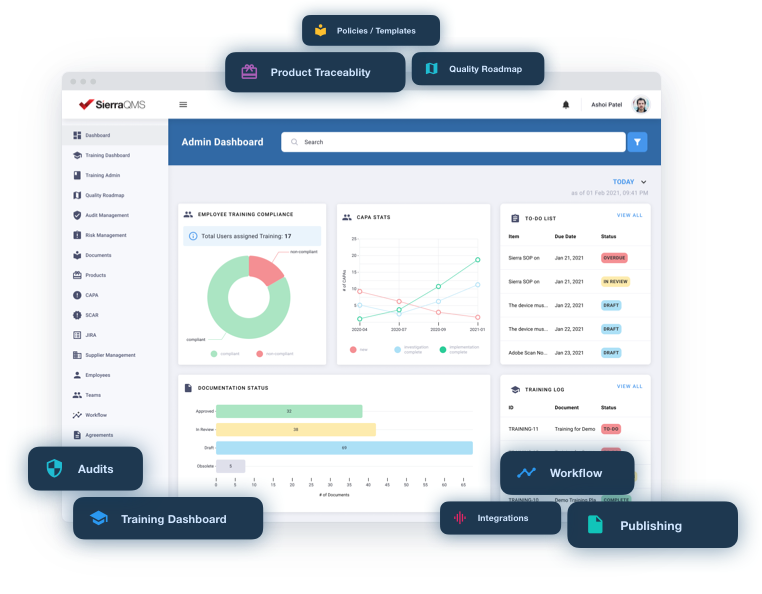
System provides full traceability and generates reports in seconds, making it easier to reach certification.
System enables your organization to stay compliant and always audit ready.

Medical Device Approval

Quality Management System

Software as a Medical Device (SaMD)

Good Manufacturing Practice (GMP)

Digital Health Certification

System and Organization Controls

European Medical Device Regulation

General Data Protection Regulation

"Sierra Labs was paramount in streamlining our quality documentation process automation. Our newly integrated QMS software has allowed TLS to have clear traceability with the ability to pull any relevant documentation when needed."
"We're a life science company developing a digital health platform that requires FDA approval. Sierra Labs has helped us get our quality management system in place and automate our compliance workflows."
"Sierra Labs removed all of the stress and confusion from HIPAA compliance, allowing us to focus on our product development. Their team configured and deployed our application into a HIPAA compliant AWS Cloud environment, drafted our policies and procedures, and aligned our current processes with HIPAA standards in just under 2 months."
"Sierra Labs is automating compliance workflows for our GMP manufacturing and laboratory facility. It has helped us reduce cost and expedite production by 3x."

Need Policies and Standard Operating Procedures (SOPs)? Use our policy generator and regulatory database. Our Library contains documentation for Medical Devices, ISO, HIPAA, Sample SOPs, FDA, EU, and more!
Try Sierra Policies NowLearn more
We can integrate with the existing tools you already use and automate the publishing of compliance and regulatory documentation. We use natural language processing (NLP) to help suggest, process, and transform your data into auditable documents.
Learn more
Sierra QMS is tightly integrated with Atlasssian’s Jira and other workflow tools your team is already using to ensure your projects maintain compliance throughout their agile lifecycle. The solution conforms to 21 CFR Part 11 requirements with electronic signatures and document export.
Learn more
Use our cloud validation product to automate the policy configuration and testing of Amazon Web Services (AWS) cloud infrastructure.
Learn moreDesigned for healthcare and life science companies of all shapes and sizes.
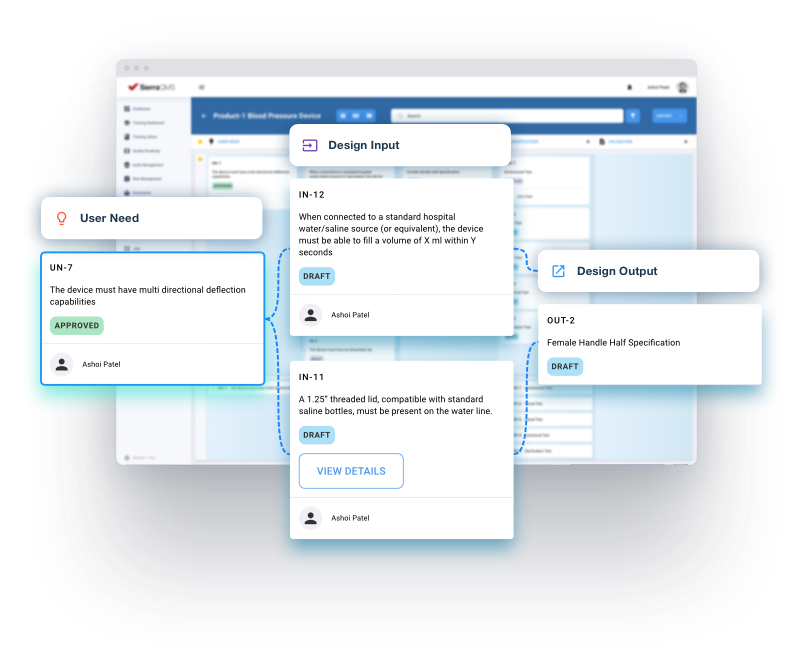
Utilize workflows designed to help you create everything needed for 510(k) submission and create quality records needed for 21 CFR Part 820, ISO 13485, SaMD 62304, and other regulatory requirements.
Track SOPs, GxP system inventories, and all components of your System/Device Life Cycle with workflows designed to comply with 21 CFR Part 11, GLP, GCP, and other industry standards.
Manage patient data electronically with a secure, HIPAA compliant system. Track non-conformance, deviations, and CAPAs with customized quality management reports that will ensure you obtain desired certifications such as HITRUST and SOC2.
Our solutions can reduce your cost by 3x
GxP Validation adds overhead. Automate validation testing on devices, apps, web, and custom off the shelf software for your enterprise. Reduce time and resources needed for lengthy testing, reporting, and approvals.
We can help decrease the time and money spent on meeting compliance and regulatory requirements.
Our automation solution generates compliance and regulatory documentation from the tools you already use.
We help bridge the gap between your IT and Operations teams with your Compliance and Regulatory teams.